Lewis structures also known as Lewis dot diagrams electron dot diagrams Lewis dot formulas Lewis dot structures and electron dot structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. This was his octet rule.
 Electron Dot Diagram Of Hydronium Download Wiring Diagrams
Electron Dot Diagram Of Hydronium Download Wiring Diagrams
The physical properties of the molecule like boiling point surface tension etc.

Lewis dot structure definition. This organic chemistry video tutorial explains how to draw lewis structures using a simple method. He described what he called the cubical atom because a cube has 8 corners to represent the outer valence shell electrons which can be shared to create a bond. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule.
Lewis dot structure definition. A drawing that shows. The major reason why learning Lewis dot structure is important is that it helps in predicting the number and type of bonds which can be formed around an atom.
A Lewis structure is a drawing of a molecule that illustrates the connectivity of the compound as well as identifying the lone pairs of electrons available for bonding with other molecules. The purpose of drawing a Lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation. They also display the total number of lone pairs present in each of the atoms that constitute the molecule.
For example chlorine with seven valence electrons is one electron short of an octet. The Lewis structure of water suggests that this molecule has nonbonding pairs of valence electrons and can therefore act as a Lewis base. A visual way to clearly depict the connection of atoms and the electrons present in a molecule.
Lewis Structures are important to learn because they help us predict. How the molecule might react with other molecules. A drawing that shows the way in which atoms are connected in a molecule 2.
Lewis structures also known as Lewis dot formulas Lewis dot structures electron dot structures or Lewis electron dot structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule as well as coordination compounds. Lewis Dot Structure Definition.
A Lewis structure can be drawn for any covalently bonded molecule as well as coordination compounds. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. A Lewis structure also helps to make a prediction about the geometry of a molecule.
In 1916 ten years before the Schrodinger wave equation G. A Lewis structure can be drawn for any covalently bonded molecule as well as coordination compounds. Lines between atoms in a Lewis structure represent covalent bonds and lone pair electrons are drawn as a pair of dots.
Using Lewis Dot Symbols to Describe Covalent Bonding The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure stoichiometry and properties. The shape of a molecule. A way of representing atoms or molecules by showing electrons as dots surrounding the element symbol.
Lewis structures also known as Lewis dot diagrams electron dot diagrams and electron dot structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. With a carbon Lewis dot structure one can see how the atoms in a molecule are bonded together which gives us more information about the structure than the molecular formula. Lewis Dot Structures - Definition and Example Lewis structure is basically a graphic representation of the electron distribution around an atom.
The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. One bond is represented as two electrons. The Lewis structure was named after Gilbert N.
Lewis who introduced it in his 1916 article The Atom and the Molecule. The electron configuration of the Al 3 ion suggests that this ion has empty 3 s 3 p and 3 d orbitals that can be used to hold pairs of nonbonding electrons donated by neighboring water molecules. Lewis structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
A Lewis structure is a graphic representation of the electron distribution around atoms. A Lewis structure is a structural representation of a molecule where dots are used to show electron positions around the atoms and lines or dot pairs represent covalent bonds between atoms. Lewis suggested that a chemical bond involved sharing of electrons.
Osmosis - 2It is the movement of water from high to low concentration. Osmosis involves solvent movements from lower to higher concentration regions while diffusion is characterized by solvent movement from higher to lower concentration regions.
 Osmosis Vs Diffusion Definition And Examples
Osmosis Vs Diffusion Definition And Examples
Substances move from a high to a low concentration down a concentration gradient.
Compare and contrast diffusion and osmosis. Osmosis is the movement of water molecules through the cell while diffusion is the movement of any molecules in and out of a cell. The intake of water in plants is an example of osmosis. Carbon dioxide oxygen water food substances wastes eg urea.
In the case of osmosis a carrier is not required in the movement of the water molecules. The faster molecules move the greater the rate of diffusion. Compare and contrast diffusion and osmosis.
On the other hand the movement can be in solid liquid or gases in the process of diffusion. 1simple diffusion- nonpolar and lipid soluble substances diffuse directly through the lipid bilayer. Simple diffusion is a type of passive transport which as the name suggests is simply the unassisted movement of solute which occurs when its electrochemical potentials on the two sides of a permeable barrier are different.
Diffusion is the movement of particles from a high to lower concentration. Furthermore osmosis requires a semi-permeable membrane while diffusion does not. This means that water is an essential for the process of osmosis to take place.
Diffusion can occur in any mixture including one that includes a semipermeable membrane while osmosis always occurs across a semipermeable membrane. The lighter the molecule the greater the rate of diffusion. Two differences and two similarities.
Water moves from a high. Active transport moves particles from low to higher concentration. This highlights a noticeable difference between osmosis and facilitated diffusion.
Facilitated diffusion is the process of biological transport in which specific structural components. Diffusion is observed when a drop of food colouring is added to a glass of water where eventually the entire water content becomes coloured. Happens as a gas disperses through small holes.
Diffusion- 1It takes place in any medium. Differences between osmosis and diffusion are - Osmosis - 1Occurs only in liquid medium. Diffusion sees molecules in an area of high concentration move to areas with a lower concentration while osmosis refers to the process by which water or other solvents moves through a semipermeable membrane leaving other bits of matter in its wake.
Osmosis undertakes in liquid medium only whereas the process of diffusion undertakes in any medium solid liquid and gases. When people discuss osmosis in biology it always refers to the movement of water. Osmosisis the diffusion of water across a semipermeable membrane.
Compare and contrast simple diffusion facilitated diffusion and osmosis relative to substances transported direction and mechanism. Basis For Comparison Diffusion Osmosis. The diffusion of water through a cell membrane.
Draw a picture of a cell in isotonic hypotonic and hypertonic states. Water is only the diffusing molecule in osmosis. Diffusion- 2It is the movement of substance from higher to lower concentration.
Osmosis requires a semi-permeable membrane while diffusion does not. Diffusion is the movement of particles from a region of high concentration to low concentration while osmosis the movement of water molecules via a semi-permeable membrane from dilute to the concentrated solution. This concept is crucial for anyone who wants to become a great clinic.
The diffusion of a large number of particles within a liquid that remain intact or interacting with other particles. The movement of solutes existing in any state of solid liquid or gas from a region of their higher concentration to the region of lower concentration but without a semipermeable membrane. Diffusion can be observed in any combination of a mixture even a partially-permeable membrane whereas osmosis is only observed across a partially permeable membrane.
Osmosis is the diffusion of water across a membrane. Osmosis is a slow process and diffusion is the fast process. Osmosis and diffusion play essential but distinct roles in the human body.
In this short video you will learn about the difference between osmosis and diffusion. Water molecules Osmosis involves movement of water molecules across a semi permeable membrane. The movement of electrons resulting in electric current.
Mariah is looking for her keys still. He is begging to save her mothers life in front of the doctor.
To better understand how helping verbs support main verbs consider the examples below.

What is a helping verb example. Here are some examples of helping verbs in sentences. To have I have three dogs. Helping verbs expressing tense.
David is writing a novel about wildlife heritage. The first example goes like this when you use To be with the main verb it will create the progressive tenses in the passive voice. Different types of helping verbs support or encourage the main verbs in different ways.
The chicken was eaten by Bonzo. I am going to the market today. Am is was are were been be being Indicates tense voice or continuity.
In this example the to be verb was forms the progressive tense -which is used for ongoing actions. For example in the sentence Shyla does not want a new bicycle the negative particle not comes between the helping verb does and the main verb want. The primary helping verbs are to do to be and to have.
Modal verbs help to show obligation possibility or necessity in a sentence. Can is the helping verb that expresses the ability of the main verb. Tomas has gone abroad.
They provide support and add additional meaning. Samara has been watching a TV. To do I did my assignment yesterday.
For instance they can show tense which indicates when an action happened ability intention or possibility. The big problem here is simply that youre First of all the distinction between strong and weak words is a grammatical one. Examples of Helping Verbs Expressing Voice Here are some examples of helping verbs expressing voice.
Helping Verb To Be. Samara was watching a TV. Actually there are more helping verbs than them.
She has a sore back. In short a helping verb can specify the link or relation between the verb action and the time tense. The helping verb is that they support the main verb and also conjugate it to create a verb tense.
Primary Helping Verb Examples. Helping verbs generally make aid and help as in their name. So what they are.
There can be up to three helping verbs in a single sentence and they can be in the negative form too. Michael had eaten the chicken before we could stop him. Sometimes a helping verb can also be used.
More than one helping verb can be used in a sentence. Samara will be watching a TV. Examples of verb in Sentences.
Modal auxiliaries or modal helping verbs dont change according to the subject. The primary helping verbs are to be to do and to have. Peter was singing for an hour.
For instance in the. A verb is the action word of a sentence. Auxiliary Verbs are those helping verbs that are used to add emphasis or express tense.
Samara is watching a TV. For example in the sentence Shyla can ride her sisters bicycle the helping verb can stands in front of ride which is the main verb. Examples of primary helping verbs in use.
All continuous tense structures have be V-ing. Depending on the subjects auxiliary verbs change their form. In each of these examples the helping verbs have and been help to form the perfect progressive tense which is the tense used for expressing an ongoing actions completion Read more about the tenses.
What are primary helping verbs. Helping verbs that perform other specific tasks to further modify the action or meaning of the main verb are called modal verbs. What is a helping verb example.
The progressive and perfect tense is commonly associated with helping verbs. The purpose of auxiliary verbs is to help the action verbs. Progressive The novel was written.
He does gymnastics daily. Examples of primary helping verbs are is was are and so on. Samara had been watching a TV.
Modal verbs do not change form. Different helping verbs help or support the main verb in different ways. A helping verb is also known as an auxiliary verb A helping verb always stands in front of a main verb.
To do to have and to be are the basic helping verbs. For example we can show tense when and where the actions are used intention possibility or ability. Helping verbs help the main verb of the sentence through modifying common verb forms of to be to do and to have.
The following helping verbs list and examples will shed some light on the usage of helping verbs in the context of rules of English grammar. V3 gone played written etc is used in all perfect tense sentences ie after have has had helping verbs. Before we continue with examples here is a list of the helping verbs in the English language.
Helping verbs sometimes called auxiliary verbs are as the name suggests verbs that help another verb. Primary helping verbs are verbs that can stand alone in a statement without a main verb. To be I am.
While its not required for all teachers to practice curving on a grade plenty of teachers in all types of formal education do this when they think its necessary to help more students pass. Constructing the Curve via the Percentages or deviations from mean The curve or grading distribution is based on the below figure shown for two different set points for the mean a weak or a strong C.
 Do You Really Want That Grade Curved
Do You Really Want That Grade Curved
The absolute values are less relevant provided that the order of the scores corresponds to the relative performance of each student within the course.
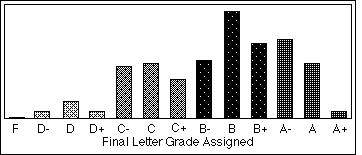
How does grading on a curve work. Traditionally grading on a curve meant normalizing the grades literally to fit the normal Bell curve. One method of grading on a curve uses three steps. Lets say theres a test with 24 questions and the best grade any student gets is a 22.
There are many reasons a teacher or professor may decide to curve a grade - for instance if the majority of students performed below what was expected which may imply that an assignment or test was out of range in either scope or difficulty. One one hand some teachers. This traditional curve takes the scores achieved by students in your class and distributes them across an even bell curve so that some students get the top grade most students get a grade somewhere in the middle and some students get the bottom grade.
In classes where the grading is curved your grade is in fact determined by your position relative to other students in the class. Numeric scores or possibly scores on a sufficiently fine-grained ordinal scale are assigned to the students. Method 1 - Mathematical curving approach.
Method 4 - Use a bell curve. When a test is being graded on a curve it might mean that everyones scores are bumped up. Grading curves are often but definitely not always based on standard deviation - for example a score equal to the class average might be a B one standard deviation SD above might be an A one standard deviation below might be a C etc.
- however it varies and this is just one example. Most of the time grading on a curve boosts the students grades by moving their actual scores up a few notches perhaps increasing the letter grade. The mean was set to a weak C based on the grade outcome and available score augmentations in the class.
It may also mean that the number of As to be rewarded is limited to a certain percentage. So teachers have to be careful about using it. Method 3 - Use a bottom limit to define an F.
My physics teacher in high school used a curve in which the highest grade was considered 100 and the other grades were multiplied by the same amount. No matter how well designed the grading system is the curving can make some students feel they are being short-changed. Say 80 was the high grade.
Grading usually is not a zero-sum game. A grading curve usually works in one of two ways which are called bell curves or distribution curves. Find out what grading curves are generally used in col.
Grading on a curve refers to the process of adjusting student grades in order to ensure that a test or assignment has the proper distribution throughout the class for example only 20 of students receive As 30 receive Bs and so on as well as a desired total average for example a C grade average for a given test. The first and simplest is to adjust what a perfect score is on a test based on the highest grade in a class. As I presented a past post titled Lets Take the Bell Curve Away from the Classroom we can think of two broad philosophies that pertain to the grading of student work.
Grading on a curve means that you as a student and your grade in an exam is relative to the grades of your classmates. Grading on a curve is a method college teachers use to make sure that grades for a test are normally distributed. What is the purpose of curve grading.
How to Grade on a Curve Why Is It Needed. A grade curve is a relative grading procedure that assigns grades for assignments based on the performance of the class as a whole. When you think of a grading curve youre probably most familiar with the kind sometimes employed on high school tests or assignments.
Theres two different things that go by the name grading on a curve. The curved grading system makes the entire class compete against each other to be at the top of the curve and therefore obtain the best grades. If its an easy test and most people get a 90 then choosing to grade on a curve means 90 is a C instead of an A which seems unfair.
Method 2 - Use a flat-scale formula. But curves are not used in. Also it only makes sense in large groups.
This is a highly relative grading scale where a C is an average and there are equal numbers of As and Fs try telling students thats what curving is. 80 54 100 so all grades are multipled by 54 Grading was then done on the usual 9284etc. Grading on a curve is a term that describes a variety of different methods that a teacher uses to adjust the scores her students received on a test in some way.
A 1000 peoples grades will usually have a nice bell curve shape.
Load your cans into the container youre using as a crucible and place the crucible inside the kiln. Today Ill be melting about 300 aluminum cans at home in my backyard foundry.
 Grant Thompson Shows You How To Melt Soda Cans Down For The Aluminum Core77
Grant Thompson Shows You How To Melt Soda Cans Down For The Aluminum Core77
The plaster and sand are a good insulator and focus the heat in one central area.
Melting point of aluminum cans. Learning how to melt aluminum can save your life when youre in need of a self-defense tool or something to trade. One reason the melting temperature is so important is because of the component failure that can occur once a metal reaches its melting temperature. This is the melting point of aluminum 66032 C 122058 F but below the melting point of steel.
Aluminum can be shaped and cold forged without any heating although heating it makes the process easier. Aluminum melting point temperature is recorded to be 66037 C or 1220666 F 93352 K. Compared with iron and copper aluminum has a rather low solubility for hydrogen at 660C liquid aluminum dissolves 069 ppm H and solid aluminum only 0039 ppm H.
Aluminum retains its toughness at very low temperatures without becoming brittle like carbon steels. With the number of soda cans floating around in garbage cans everywhere you wont have to look very far to start melting. For instance creep-induced fractures can occur before the melting point is reached.
Fire up the kiln or furnace to 1220F. How to Melt Aluminum Cans at Home with a Mini Metal Foundry. AlF 3 melts at 1290 C 2354 F and is made by reaction of aluminium oxide with hydrogen fluoride gas at 700 C 1300 F.
Once you have pure aluminum you can mold it into fun or useful shapes. The melting point of aluminum cans is the same as the melting point for aluminum which is 66032C 122058F. As far we know aluminium cans are made of not pure aluminium so take my answer above just as a refference data.
To cast aluminum youll need a way to get it hot enough that it reaches its melting point of 660 degrees C 1200 degrees F Then you can either pour it into molds to create ingots or cast it to create tools and other objects. Aluminum has a low melting point so you can easily melt it using a propane torch or a DIY foundry. Grant Thompson decided to build a homemade metal foundry from a mixture of 50 plaster of paris and 50 play sand.
I have been saving these beer and soda cans from some time and Ill turn them i. Melting Point of Metals Alloys Metal Melting Point oC oF Admiralty Brass 900 940 1650 1720 Aluminum 660 1220 Aluminum Alloy 463 671 865 1240 Aluminum Bronz. Metal failure may happen before the melting point but when a metal reaches its melting temperature and begins to become a liquid it will no longer serve its intended purpose.
Youll get about 1 pound of aluminum for every 40 cans. Note some failure can occur before the metal reaches its melting temperature. The only dissolved gas in aluminum melts is hydrogen because it does not form compounds with aluminum as other gases eg.
Aluminum has high reflectivity and can be used for decorative applications. Melting aluminum cans yields pure aluminum that you can use for other projects. The melting point is important because of the component failure and the associated hazards that can happen once a metal reaches its melting point.
A substances melting point is an essential physical property. Whereas the meting point of aluminum oxide or alumina is comparatively higher than its pure metal form about. Nitrogen forms AIN oxygen forms AI 2 O 3.
What is the Purpose of Determining the Melting Point of a Substance. With heavier halides the coordination numbers are lower. It will melt aluminum cans with over 1000 degrees of heat provided by regular old charcoal and a hair dryer.
Aluminum is a good conductor of heat and electricity. Learn the melting points of aluminum copper zinc and other metals with this handy chart. The extraction is done by electrolysis but first the aluminium oxide must be melted so that electricity can pass through it.
Some aluminum alloys can match or even exceed the strength of common construction steel. The melting point of aluminum is higher when compared to other commonly occurring metals like iron copper brass magnesium zinc etc. At periodical table aluminium melting point is 93347 K or 66032 C or122058 F.
But when the metal begins to turn. The melting point of aluminum is 659 degrees Celsius or 1218 Fahrenheit. However aluminium oxide has a very high melting point over 2000C.
Types of Classification of Data in Statistics. As data is an invaluable source of business insight the knowing what are the various qualitative data analysis methods and techniques has a crucial importance.
 Qualitative And Quantitative Data Infographic And Examples Data Science Qualitative Research Methods Research Skills
Qualitative And Quantitative Data Infographic And Examples Data Science Qualitative Research Methods Research Skills
There are different approaches types of statistical methods strategies and ways to analyze qualitative data.

Qualitative data examples statistics. For example think of a student reading a paragraph from a book during one of the class sessions. Examples of Qualitative Data. Qualitative data is descriptive data like color taste texture smell etc.
Qualitative data describes a subject and cannot be expressed as a number. Qualitative data are measures of types and may be represented as a name or symbol. For example you might be working with interview transcripts survey responses fieldnotes or recordings from natural settings.
Examples Amount of money you have Height Weight Number of people living in your town Number of students who take statistics. For example answers like yes or no to a question or the sex of participants male or female are among the most common nominal data. Variable qualitative ordinal.
There are two types of quantitative data continuous and discrete. Recall the difference between quantitative and qualitative data. Quantitative data is a set of numbers collected from a group of people and involves statistical analysisFor example if you conduct a satisfaction survey from participants and ask them to rate their experience on a scale of 1 to 5.
Qualitative data are the result of categorizing or describing attributes of a population. Most types of qualitative data analysis share the same five steps. Ordinary qualitative variables are known as semi-quantitative variables.
Qualitative data can take the form of texts photos videos and audio. Quantitative data is analyzed using statistical analysis while qualitative data is analyzed by grouping it in terms of meaningful categories or themes. Qualitative or Categorical Data.
The data in Statistics are classified as follows. Qualitative data analysis is very important because it allows data sciences and statisticians to form parameters for observing and analyzing larger sets of data. Statistics that describe or summarize can be produced for quantitative data and to a lesser extent for qualitative data.
Let us discuss the different types of data in Statistics herewith examples. Data that you will see. As all of these question types collect data that fit into set categories or can be calculated into averages and other statistics they are quantitative.
Quantitative data can be expressed as numbers. Quantitative data on the other hand is data that is measurableFor example qualitative data of milk might discuss its a white liquidQuantitative data of the same milk might discuss there are 3 gallons of milk. Probably the most common qualitative variable in scientific research at least when we analyze aspects related to human behavior and health.
During the voting process we take nominal data of the candidate a voter is voting for. Color unless it can be written as a specific wavelength of light expressions of moreless uglybeautiful fatthin healthysickly. See another example to drive this difference home.
Quantitative data defines a subject and is expressed as a number it can be quantified that can be analyzed. Qualitative data are generally described by words or letters. It is often compared to quantitative data information that is expressed with a numberGenerally speaking qualitative data is higher value than quantitative data but it cant be easily processed by machines.
The difference between quantitative and qualitative data. A teacher who is listening to the reading gives feedback on how the child read that paragraph. Examples of qualitative data include.
An example of this type of variables can be the result of a sport competition first second or third place. Qualitative data are not numerical. The frequency of votes incurred by each candidate is measured and the candidate with the highest number of votes is made the winner.
Qualitative data is also called categorical data since this data can be grouped according to categories. The hair colors of players on a football team the color of cars in a parking lot the letter grades of students in a classroom the types of coins in a jar and the shape of candies in a variety pack are all examples of qualitative data so long as a particular number is not assigned to any of these descriptions. For example if a company need to identify the diversity of its personnel it would look at qualitative data such as ethnicity and race of its employees.
For comparison quantitative data in this case could be the frequency of workers to belong to those ethnicities and races. Quantitative data is gathered by measuring and counting. Prepare and organize your data.
In general the qualitative data analysis has the. In short the moniker demands this data be organized or separated by name only. Examples of Quantitative Data.
Qualitative data is information expressed in a language such as English. Qualitative data also known as the categorical data describes the data that fits into the categories. Qualitative data is collected by interviewing and observing.
Below you will find a series of examples of typical qualitative variables although it must be taken into account that it is often possible to make a variable of this type operational and quantitative. There are two types of data that we can collect. A definition of qualitative data with examples.
Qualitative Data Examples in Statistics. In statistical terms we call this mode. Quantitative data are always numbers.
Quantitative data are data about numeric values. Although they allude to attributes or qualities that lack a numerical value they are classified within a scale of value. Qualitative data can also be collected through certain types of survey questions in addition to interviews and focus groups.
Nominal variables are qualitative data statistics that have no order or sequential ranking.
HClO4 perchloric acid Weak. If an acid is not listed here it is a weak acid.
 Tips For Identifying Acid And Base Strength Concept Chemistry Video By Brightstorm
Tips For Identifying Acid And Base Strength Concept Chemistry Video By Brightstorm
Strong acids are not named as such because they are more powerful than other acids.
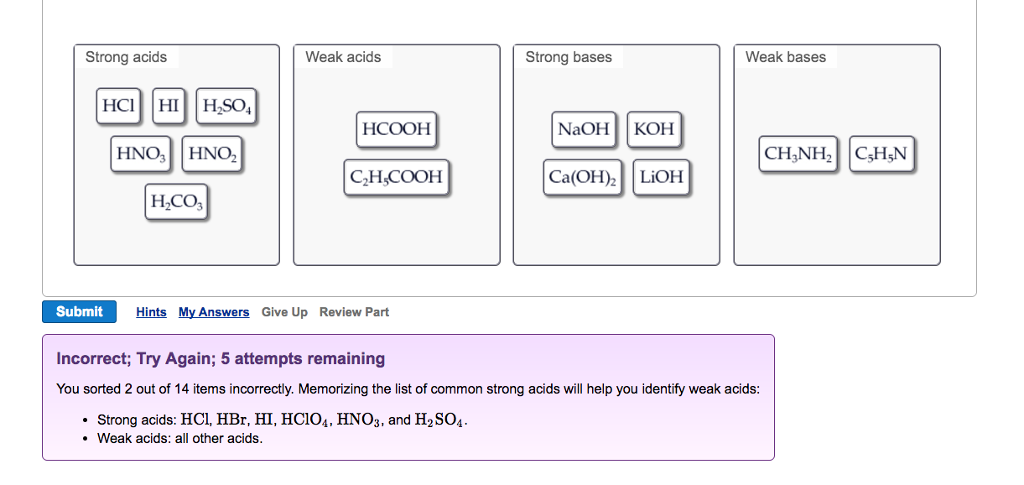
List of strong acids. There are 7 strong acids. The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table. Any acid that dissociates 100 into ions is called a strong acid.
It may be 1 ionized or 99 ionized but it is still classified as a weak acid. Though it is not necessary for this module it is helpful to memorize the strong acids and bases for chemistry courses at the university level. The strongest acids are at the bottom left and the strongest bases are at the top right.
Identify each acid or base as strong or weak. The conjugate base of a strong acid is a very weak base and conversely the conjugate acid of a strong base is a very weak acid. Strong Acids and Bases Pop up List Here is a list of strong acids and bases that completely dissociate in aqueous solutions.
Hydrogen chloride is described as a strong acid. The term strong in the name refers to the acids ability to release hydrogen H molecules which allows it to become ionized when placed into a solution of water. This cheat sheet below is meant to accompany the MCAT AcidBase Tutorial Series math focus as well as the Orgo AcidBase Tutorial series trendsconcept focus.
Acid with values less than one are considered weak. A strong acid is one which is virtually 100 ionised in solution. Strong acids like HCl or HNO 3 are such good proton donors that none of their own molecules can remain in aqueous solutionAll HCl molecules for example transfer their protons to H 2 O molecules and so the solution contains only H 3 O aq and Cl aq ions.
Examples of Strong Acids. In general the pKa of the strong acid the acidity coefficient the negative log of the ionization constant is less than 1. Hydrochloric acid denoted by the chemical formula HCl Hydrobromic acid denoted by the chemical formula HBr Hydroiodic acid or hydriodic acid denoted by the chemical formula HI.
What makes them strong is the fact that they completely dissociate into their ions H and an anion when they are mixed with water. Weak acids do not have this ability. Because HCl is listed in Table 122 Strong Acids and Bases it is a strong acid.
For a list of common weak acids and bases see Table 8-2 in Oxtoby8-2 in Oxtoby. The only weak acid formed by the reaction between hydrogen and a halogen is hydrofluoric acid HF. Every other acid is a weak acidBecause there are only seven strong acids it is easy to commit the list to memory.
Perchloric acid HClO 4. CH3COOH acetic acid HCOOH formic acid HF hydrofluoric acid HCN hydrocyanic acid HNO2 nitrous acid HSO4- hydrogen sulfate ion. As it turns out there are very few strong acids which are given in Table PageIndex1.
The nitrogen in C 5 H 5 N would act as a proton acceptor and therefore can be considered a base but because it does not. The most straight-forward examples involving acids and bases deal with strong acids and bases. Strong acids mainly refer to permanganic acid hydrochloric acid hydrochloric acid sulfuric acid nitric acid perchloric acid selenoic acid hydrobromic acid hydroiodic acid and chloric acid.
In chemistry there are seven strong acids. There are seven strong acid. Other common strong acids include sulphuric acid and nitric acid.
MgOH 2 C 5 H 5 N. The list of strong acids is provided below. While technically a weak acid hydrofluoric acid is extremely powerful and highly corrosive.
Because MgOH 2 is listed in Table 122 Strong Acids and Bases it is a strong base. The strong acids are hydrochloric acid nitric acid sulfuric acid hydrobromic acid hydroiodic acid perchloric acid and chloric acid. List of Strong Acids Bases in Order.
Some common strong acids acids with pKa -1 include. HCl hydrochloric acid HNO3 nitric acid H2SO4 sulfuric acid HBr hydrobromic acid HI hydroiodic acid. Strong acids are listed at the top left hand corner of the table and have Ka values 1 2.
Strong acids like strong bases can cause chemical burns when exposed to living tissue. As we explained in the preceding lesson all. A strong acid you will recall is one that is stronger than the hydronium ion H 3 O This means that in the presence of water the proton on a strong acid such as HCl will fall into the sink provided by H 2 O converting the latter into its conjugate acid H 3 O In other words H 3 O is the strongest acid that can exist in aqueous solution.
Chloric acid hydrobromic acid hydrochloric acid hydroiodic acid nitric acid perchloric acid and sulfuric acid. List of Strong Acids. A strong acid is one that dissolves in water.
Being part of the list of strong acids doesnt give any indication of how dangerous or damaging an acid is though. The Relative Strengths of Some Common Conjugate AcidBase Pairs. If it does not dissociate 100 it is a weak acid.
Strong Acids HCl - hydrochloric acid HNO 3 - nitric acid H 2 SO 4 - sulfuric. Youll not only have to recognize the weak acids and bases but MEMORIZE the standard list of strong acids and bases listed on the cheat sheet below.
