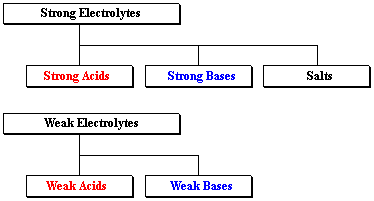
Strong electrolytes including strong aci. While weak electrolytes are divided into weak acids and weak bases.
Look Back Through The Discussion Of Electrolytes And Table 3 2 And Identify At Least One Strong Electrolyte One Weak Electrolyte And One Nonelectrolyte Different From Those Discussed In Problem Solving Example 3 7 Table
Weak electrolytes ionize partially about 1 to 10 in water.

Strong and weak electrolytes. A weak electrolyte is a solute that partially dissociates or separates into its constituent cations and anions during the solvation process. Ben Mills Weak electrolytes partially ionize in water. Therefore HF is a weak electrolyte.
Learn vocabulary terms and more with flashcards games and other study tools. Hydrochloric acid HCl and acetic acid HAc were chosen for our strong and weak electrolytes respectively. Strong acids strong bases and salts are strong electrolytes.
Strong electrolytes dissociate completely at moderate concentrations. Difference Between Strong and Weak Electrolytes Definition. A weak electrolyte is a poor conductor of electricity.
Using these two solutions proved useful to show that strong electrolytes are stronger conductors than weak electrolytes. Strong acids HNO 3 aq H 2 SO 4 aq HCl aq a. Generally the component present in the greatest quantity is the solvent.
Strong electrolytes follow Kohlrauschs law whereas weak electrolytes are described by Ost-walds dilution law. Strong electrolytes completely dissociate or ionize in a solution whereas a weak electrolyte partially dissociate or ionize. In this experiment the differing behaviors of strong and weak electrolytes with regard to conductivity was studied.
Strong and Weak Electrolytes. Strong acids strong bases and salts. Strong and weak electrolytes by measuring their electrical conduc-tance.
Examples of bases that are weak include ammonia and many more. We explain Strong and Weak Electrolytes with video tutorials and quizzes using our Many WaysTM approach from multiple teachers. The examination of the concentration dependence of the conductivity allows the molar conductivities of infinitely diluted electrolytes to be determined and.
Strong electrolytes ionize completely 100 in water. Most compounds that contain nitrogen are weak electrolytes. H 2 O - water weakly dissociates in itself.
Strong and weak electrolytes. HF - hydrofluoric acid. However the cations and anions that result from the solvation of a weak electrolyte experience strong electrostatic attractions and often recombine to regenerate the solute molecule from which they were produced.
Strong electrolytes can completely dissociate into ions. This chemistry video tutorial explains how to identify weak electrolytes strong electrolytes and nonelectrolytes. Electrolytes - A substance that when dissolved in water produces a solution that can conduct an electric current.
This lesson will explain that some solutes dissociate more readily than others in aqueous solutions and that the greater the ability the stronger the electrolyte. The little amount of ethanoate makes acetic acid a weak electrolyte rather than a strong electrolyte. NH 3 - ammonia.
Strong electrolytes readily dissolve in water but weak electrolytes do not readily dissolve. CH 3 CO 2 H - acetic acid. Weak acids organic acids--acetic citric butyricmalic etc b.
All ionic compounds are strong electrolytes because they separate into ions when they dissolve in water. Weak electrolytes are compounds that partially dissociate into its ions when dissolved in water. Start studying Strong and weak electrolytes.
Weak electrolytes include weak acids weak bases and a variety of other compounds. Pretty much any dissociation into ions between 0 and 100 makes a chemical a weak electrolyte but in practice around 1 to 10 of a weak. Strong and weak electrolytes can also be applied to bases and other compounds that dissolve in water to produce ions.
What is the difference between Strong Electrolytes and Weak Electrolytes. In a solution a solute is a substance that dissolves in a solvent. A strong electrolyte exists mainly as ions in solution.
Strong electrolytes are compounds that can completely dissociate into its ions when dissolved in water. A weak electrolyte has only a small amount of ionization in solution. Weak electrolytes will be electrolytes that dont separate into particles in arrangement and just mostly ionize in arrangement about 1-10.
Strong electrolytes are divided into three categories. Weak electrolytes only partially break into ions in water. A strong electrolyte is a good conductor of electricity.
Examples of bases that are strong electrolytes include sodium hydroxide and many more. Water is the most common solvent because of its ability to dissolve almost any substance over time. The strength of an electrolyte whether it is a strong electrolyte or a weak electrolyte depends on the substances ability to form ions by dissociation or ionization.
On the other hand Strong electrolytes will be electrolytes that separate completely into particles in arrangement and just mostly ionize in arrangement about 100. Ammonia is a weak base and a weak electrolyte.
 What Are Examples Of Weak Electrolytes Quora
What Are Examples Of Weak Electrolytes Quora
 Is Carbonic Acid Is A Strong Electrolyte Quora
Is Carbonic Acid Is A Strong Electrolyte Quora
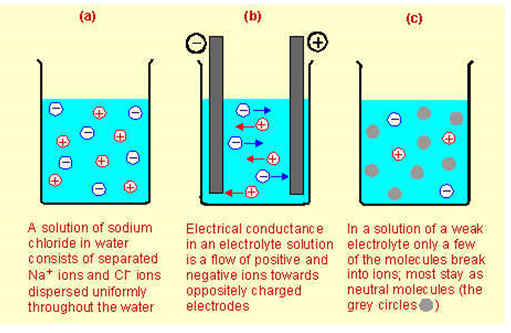 Compounds That Conduct Electricity In Solution Or When Molten Because Their Ions Are Free To Move Around Are Called Electrolytes In Solution Or Molten Ions Separate From Each Other And Move Independently Throughout The Solution Strong Electrolytes Are
Compounds That Conduct Electricity In Solution Or When Molten Because Their Ions Are Free To Move Around Are Called Electrolytes In Solution Or Molten Ions Separate From Each Other And Move Independently Throughout The Solution Strong Electrolytes Are
 How To Identify Strong Weak And Non Electrolytes Examples Practice Problems Youtube
How To Identify Strong Weak And Non Electrolytes Examples Practice Problems Youtube
Difference Between Strong And Weak Electrolytes Definition Properties Reactions
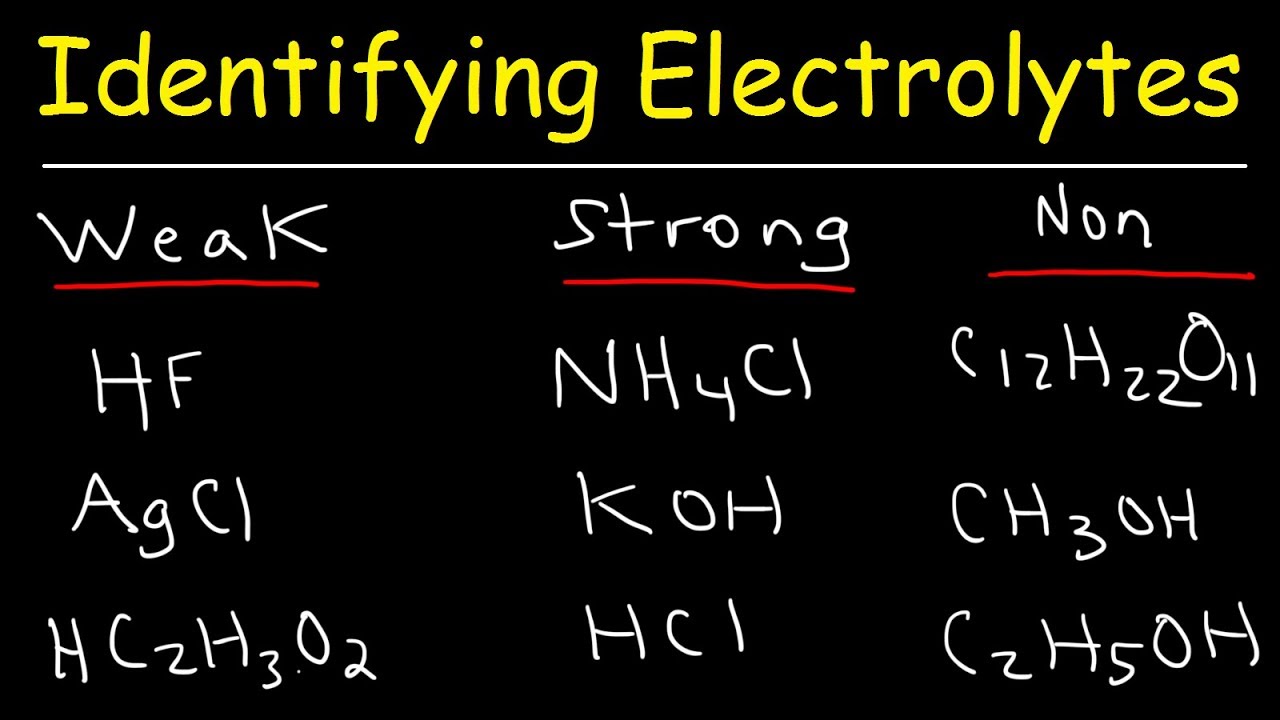 Identifying Strong Electrolytes Weak Electrolytes And Nonelectrolytes Chemistry Examples Youtube
Identifying Strong Electrolytes Weak Electrolytes And Nonelectrolytes Chemistry Examples Youtube
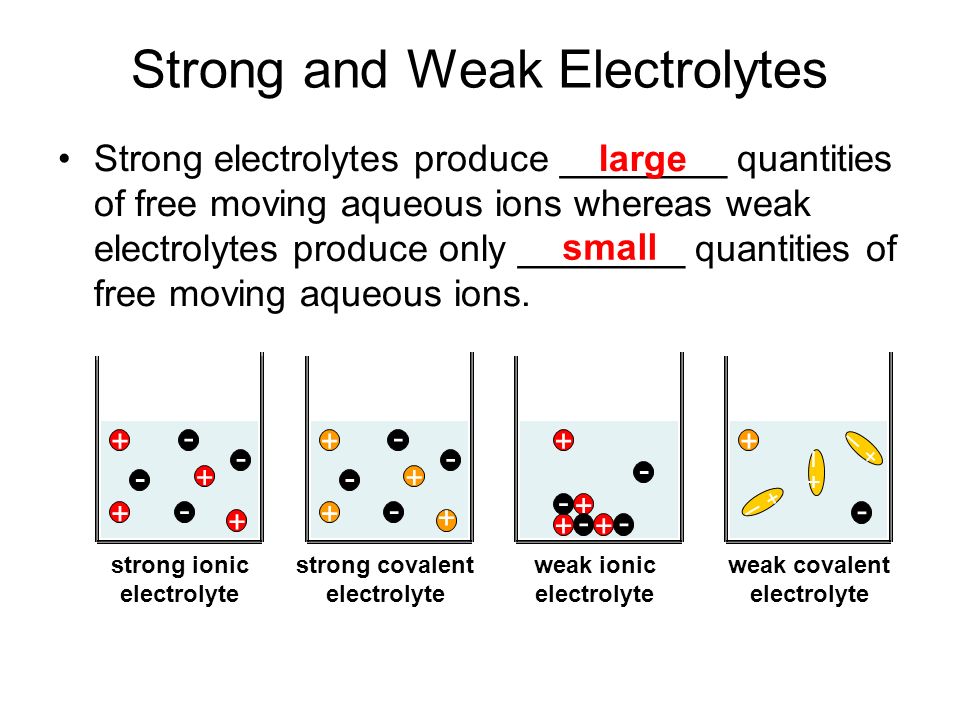 Strong And Weak Electrolytes Ppt Video Online Download
Strong And Weak Electrolytes Ppt Video Online Download
 Major Differences Strong Electrolytes Vs Weak Electrolytes
Major Differences Strong Electrolytes Vs Weak Electrolytes
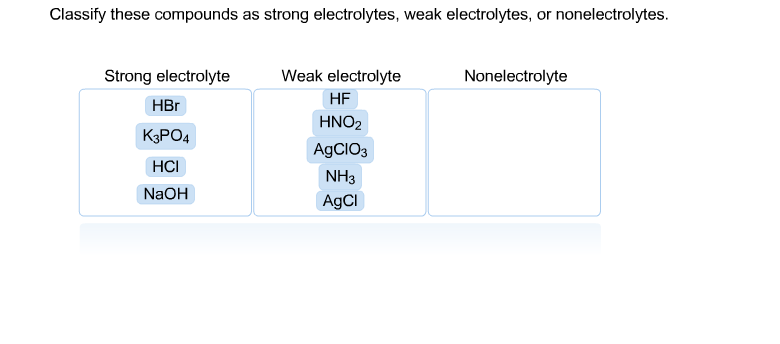 Solved Classify These Compounds As Strong Electrolytes W Chegg Com
Solved Classify These Compounds As Strong Electrolytes W Chegg Com
 Distinguish Between Strong And Weak Electrolytes And Provide Two Examples Of Each Brainly In
Distinguish Between Strong And Weak Electrolytes And Provide Two Examples Of Each Brainly In
 Strong And Weak Electrolytes Jacksonville High
Strong And Weak Electrolytes Jacksonville High
 Strong Electrolytes Vs Weak Electrolytes What Is The Difference Diffzi
Strong Electrolytes Vs Weak Electrolytes What Is The Difference Diffzi